[1]A Feasibility and Safety Study of a Novel CD19-Directed Synthetic T-Cell Receptor and Antigen Receptor (STAR) T-Cell Therapy for Refractory and Relapsed (R/R) B Cell Acute Lymphoblastic Leukemia (B-ALL). Blood. November 5, 2020.
科學創新
HJC黄金城擁有完整細胞藥物開發平臺,滿足多種臨床需求
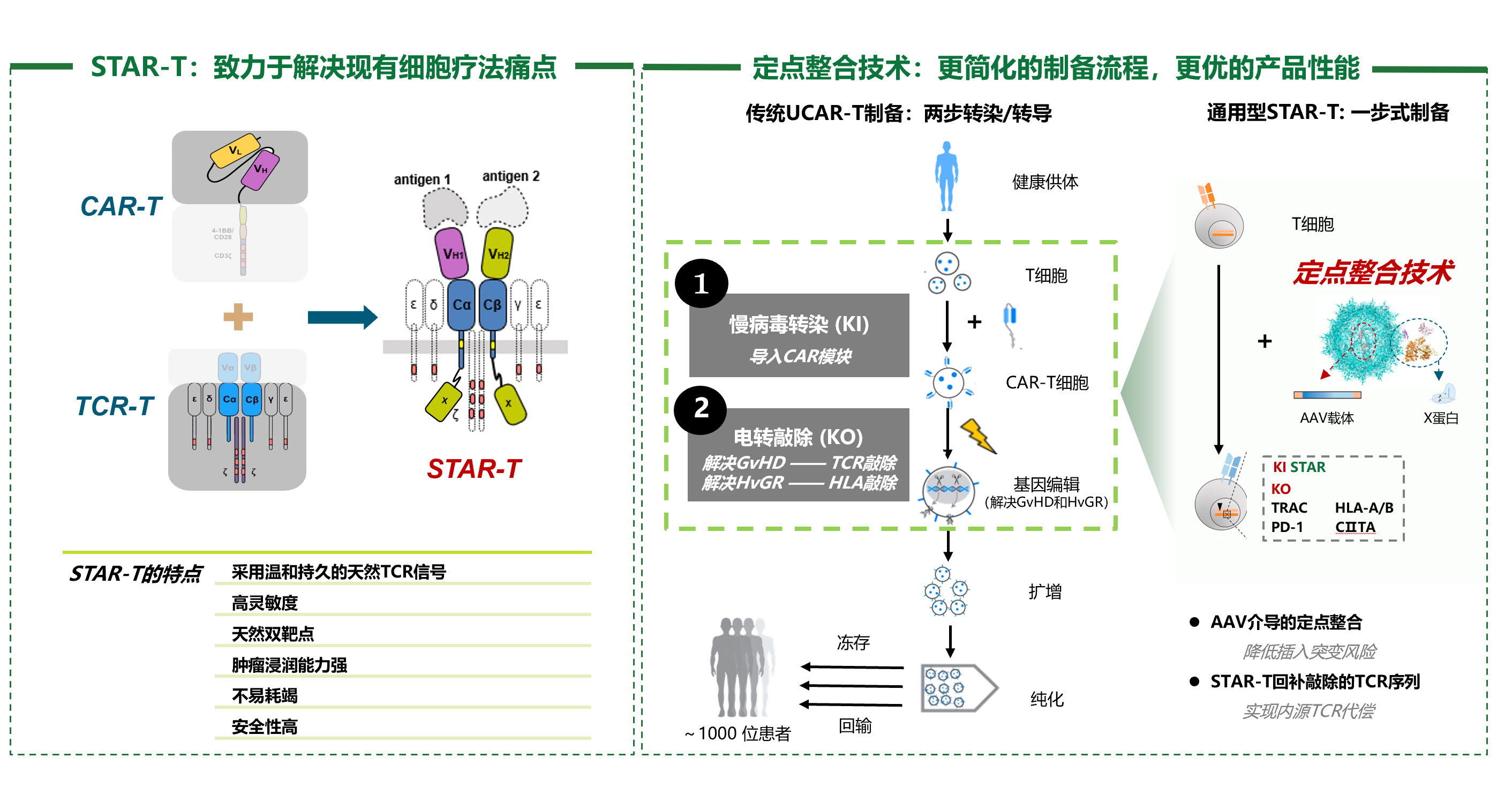
STAR-T平臺
具有自主知識產權的合成性T細胞受體抗原受體( Synthetic TCR and Antigen Receptor), STAR-T細胞治療技術平臺。
與當前細胞治療領域常用的CAR-T技術相比,STAR-T更具有天然T細胞的特性,具有天然雙靶點,毒性低,耗竭慢,浸潤性強的特點,更有可能實現對實體瘤治療的突破。
定點整合技術
自研的定點整合技術是一種基因編輯 T細胞的顛覆性突破技術,可通過非電轉體系在指定位點插入目的基因,該技術對T細胞損傷小、易于工業化放大、成本低。
其結合STAR平臺的定點整合可代償內源TCR被敲除的缺陷,開發革命性的通用型T細胞產品。